 August 26, 2011 — An open-label phase 1/2 trial of patients with treatment-resistant fibromyalgia shows that the therapy is safe and effective and may be a useful treatment addition.
August 26, 2011 — An open-label phase 1/2 trial of patients with treatment-resistant fibromyalgia shows that the therapy is safe and effective and may be a useful treatment addition.
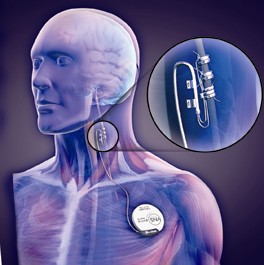
The aim of the uncontrolled single-center pilot study was to determine whether periodic but continuous stimulation of the left vagus nerve is a safe, tolerable, and useful adjunct treatment for patients reporting continued severe pain despite receiving current best medical management.
The research was led by Gudrun Lange, PhD, from the Department of Radiology, New Jersey Medical School, Newark, and carried out at the Pain and Fatigue Study Center, Department of Pain Medicine and Palliative Care, Beth Israel Medical Centre, New York City.
The results were published online August 3 in the journal Pain Medicine.
The study included 14 adult women who had physician-diagnosed fibromyalgia for at least 2 years and were refractory to conventional pharmacological treatment (ie, nonsteroidal anti-inflammatories, tricyclic antidepressants, and anticonvulsants).
The women were surgically implanted with a vagus nerve stimulation (VNS) device. After 2 weeks of recovery, they began a 2-week stimulation adjustment period during which VNS intensity was increased to deliver as high a current as could be comfortably tolerated.
All study participants tolerated the implantation. Of these, 12 patients completed the initial 3-month study, and 11 participated in a longitudinal study lasting an additional 8 months.
Same Adverse Effects
In general, the patients had the same types of adverse effects to VNS as those reported in patients with treatment-resistant epilepsy and depression, including stimulus-bound voice alteration, neck pain, nausea, and dyspnea. In addition, patients reported dry mouth and fatigue. During the study, there were 4 unanticipated/serious adverse events occurring in 4 patients.
The primary efficacy outcome was minimal clinically important difference (MCID+) that includes pain, overall wellness, and physical function. At 3 months, 5 of the14 participants became MCID+, and 2 no longer fulfilled fibromyalgia diagnostic criteria for pain and tenderness.
In addition, the therapeutic effect seemed to increase beyond the acute trial. At the end of 11 months, 7 patients were MCID+, and there was parallel improvement in fibromyalgia caseness, defined as widespread pain and at least 11 tender points, which was added as a secondary outcome.
“[W]e realized that using loss of [fibromyalgia] caseness as an outcome variable might be useful for clinicians in judging the potential efficacy of VNS,” the authors write.
The researchers were “surprised” by the robustness and ubiquity of response to the VNS treatment, they note. Although improvement in tender point threshold appears to be a difficult outcome to achieve, “our results suggest that [fibromyalgia] treatment can reduce tender point threshold to the degree that the point treated is no longer tender,” they write.
However, they add, tender point count was not a reliable predictor for continued therapeutic success.
An “obvious question” is whether the results represented a placebo effect “related to being in a treatment trial necessitating surgery, feeling a sensory stimulus throughout the day and having high hopes for a good therapeutic outcome,” said the authors. The continued improvement shown by some patients and the fact that more patients attained outcome criteria over time argues against a nonspecific or placebo explanation for the therapeutic benefit, they write.
The authors conclude that a controlled trial is needed to better understand the role of VNS in fibromyalgia.
This work was supported by a grant from the National Institutes of Health. Dr. Lange reports a US patent pending in her name for the use of VNS in fibromyalgia.
Pain Med. Published online August 3, 2011. Abstract